Table of Contents
Some (most) of the chemical formulae in this post do not have the state as a subscript (for example, $\ce{H+(g)}$ instead of $\ce{H+_{(g)}}$). This is because the IUPAC Guidelines for chemical equations recommend that:
As the science sections of the site have around 4000 users a month from outside of NSW, we prefer to stick to international industry standards when working with math and science. For these readers, this note is of no relevance.
However, the majority of our userbase is still HSC students, so we feel that its important to make note of the fact that NESA believes that they know better than the IUPAC guidelines and, as a result, in tests, you must use BOTH parentheses (brackets) AND subscript. If you are transcribing our content into your book, we recommend that you get into the habit of this.
What is an Acid?
- Acids are a group of commonly used substances with several common properties:
- Produce $\ce{H+}$ when dissolved in water
- Ionise when dissolved
- $pH\lt7$
- Sour taste
- Corrosive
- Turn blue litmus paper red
- Conduct electricity (stronger acids are better conductors)
What is a Base?
- Bases are another group of substances with common properties:
- Taste bitter
- Slippery feel (like soap)
- Produce $\ce{OH-}$ ions when dissolved on water
- $pH\gt7$
- Corrosive (caustic)
- Turn red litmus paper blue
Indicators
Indicators are a category of substances which give a qualitative indication of the pH of a solution
Indicators change color as a response to the surrounding $H^+$ and $OH^-$ ions
An acidic solution has a high concentration of hydrogen ions, while basic solutions have high concentration of Hydroxide ions
pH can be calculated quantitatively using the formula:
$$pH=-\log_{10}[\ce{H+}]$$
How do indicators work?
- Most indicators are organic weak acids or weak bases. They are a special case, in which:
$$\ce{HInd_{(aq)}<=>H+_{(aq)} +Ind-_{(aq)}}$$
$$\text{Molecule (Color 1)}\ce{<=>}\text{Hydrogen Ion + Anion (Color 2)}$$
Note: $Ind$ is an abbreviation for “indicator” and represents the bit that changes color.
- The two forms of the substance are distinctly different colors (1 and 2).
- The more $[\ce{H+}]$ changes, the more the equilibrium will shift (and so the more the color will change)
Low pH and high pH
- Lower pH means a greater concentration of Hydrogen ions
- Equilibrium tends to the left hand side
- The “hydrated” form of the indicator $(HInd)$ will dominate, so color 1 will be visible the most
- High pH means a low concentration of Hydrogen ions
- Equilibrium will tend towards the right hand side
- The “dehydrated” form of the indicator $(Ind^{-})$ will dominate, so color 2 will be visible the most
Indicator Table
| Indicator | Color at Low pH | Color at high pH | Color Change pH Range |
|---|---|---|---|
| Methyl Orange | Red | Yellow | 3.1-4.4 |
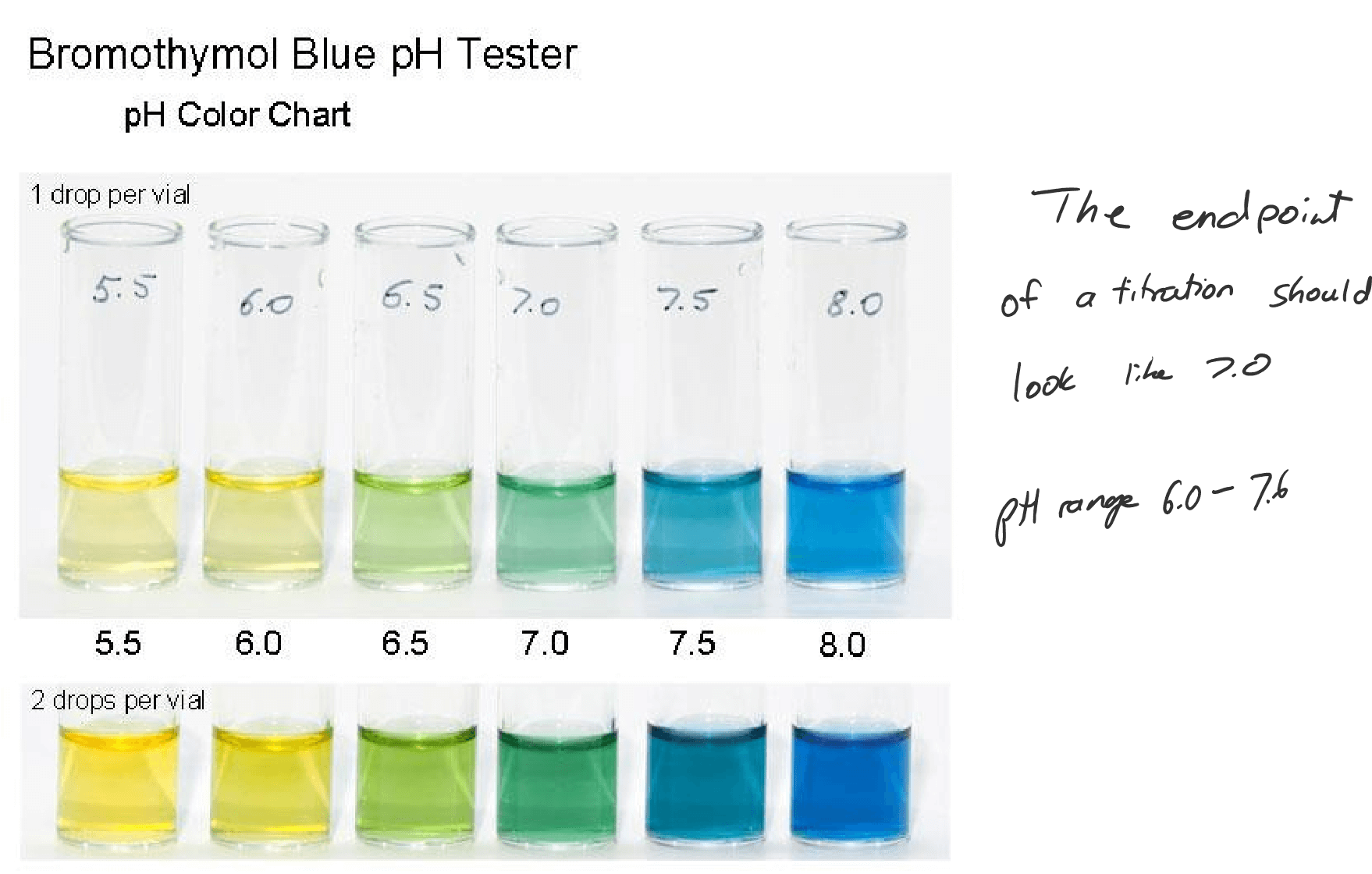
| Bromothymol Blue | Yellow | Blue | 6.0-7.6 |
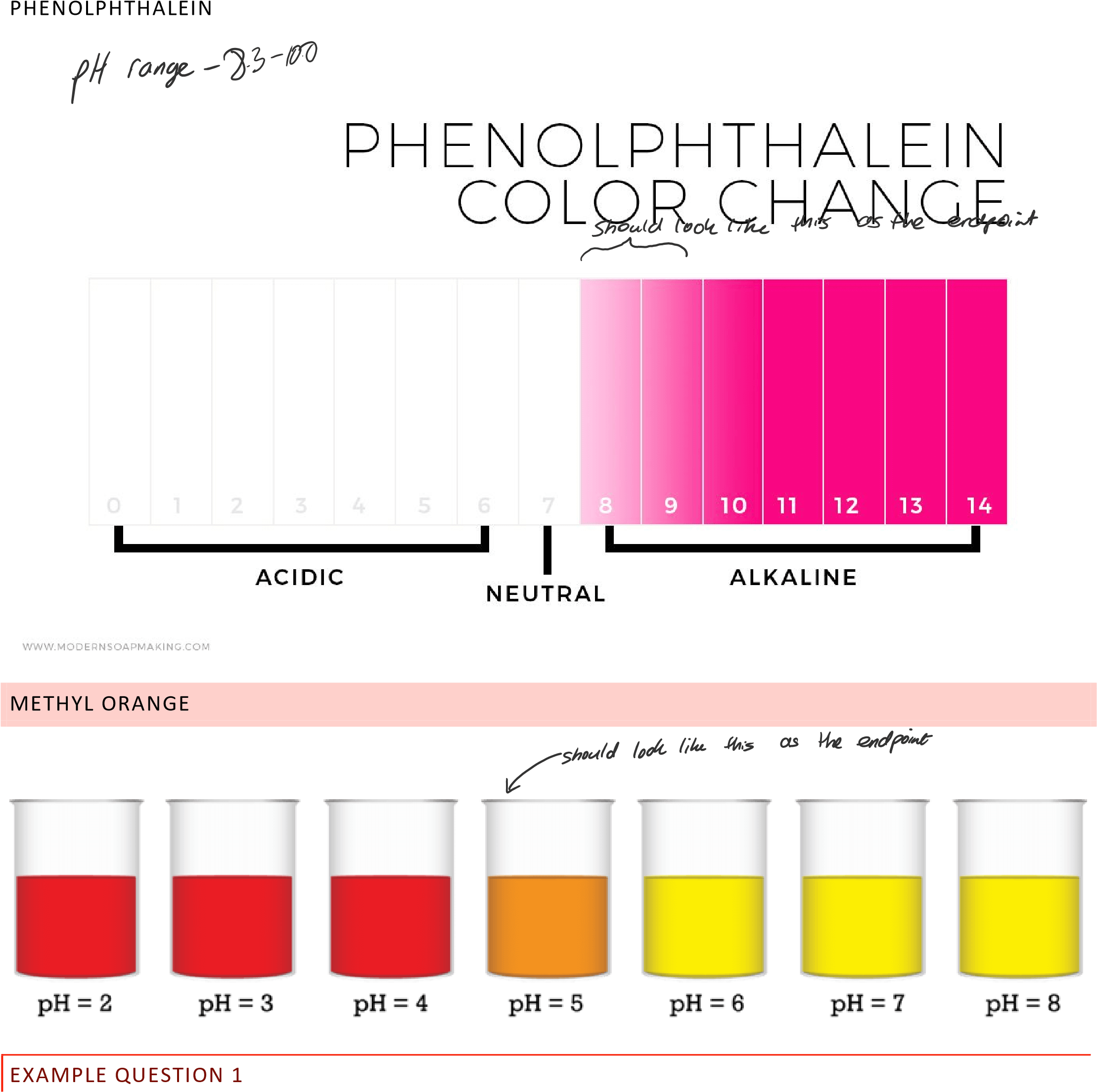
| Phenolphthalein | Colorless | Pink | 8.3-10.0 |
| Methyl Red | Red | Yellow | 4.8-6.0 |
| Litmus | Red | Blue | 4.5-8.3 |
| Phenol Red | Yellow | Red | 6.8-8.4 |
Limitations of Indicators
- pH is an approximation (not accurate)
- Cannot distinguish between strong and weak acids/bases
- Destroys or contaminates solutions
Advantages of Indicators
- Cheap and easy to use
- Provides a rudimentary indication of acidity/basicity
Everyday uses of Indicators
Swimming Pools
Swimming pools need to be maintained close to $pH=7.4$ to avoid irritation of the eyes (which are about pH 7.4)
Bromothymol Blue or Phenol Red can be used to test the pH of a pool
Acids such as Hydrochloric Acid can be used to lower the pH
Kinda weird that you can buy concentrated strong acids in a 25L container from a pool shop and no one will question it 🤷♀️ like they’re not even expensive or anything
Substances such as sodium carbonate $(\ce{Na2CO3})$ can be added to raise the pH if it’s too low
Reactions of Acids
Acid + Metal Hydroxide/Oxide (Neutralization Reaction)
Metal oxides are also bases, and react with acids to form a metal salt and water. This can be generalised as:
$$\ce{HX_{(aq)} +MOH_{(s/aq)}->MX_{(aq)} +H2O_{(l)}}$$
Ammonia is a special case of a base that will undergo neutralisation but will not produce water, as it does not contain oxygen in its formula
- Ammonia $(\ce{NH3})$ is covalent molecular
- It is a gas at room temperature and highly soluble at STP
- Instead of producing water, it produces ammonium salt as the product:
$$\ce{HBr_{(aq)} +NH3_{(aq)}->NH4Br_{(aq)}}$$
Acid + Metal Carbonate / Hydrogen Carbonate
$$\ce{HX_{(aq)} +MCO3_{(s)} ->MX_{(aq)} +H2O_{(l)} +CO2_{(g)}}$$
Acid + Reactive Metal (Redox Reaction)
$$\ce{HX_{(aq)} +M_{(s)}->MX_{(aq)} +H2_{(g)}}$$
Everyday Uses of Acids/Bases
- Bee stings and ant bites are acidic in nature. They can be neutralised using alkaline medicine such as baking powder.
- Wasp stings are alkaline in nature. Vinegar can be used to cure wasp stings because vinegar can neutralise the sting.
- When stung by a stingray, concentrated vinegar can be used to stop the nematocysts from firing off such that you won’t get injected with more venom.
Theories of Acids and Bases
Lavoisier - “Father of Chemistry” - 1776 - (Oxides)
- Antoine Lavoisier was a French chemist who established the quantitative science of chemistry
- He investigated oxides of different elements, and found that non-metal oxides reacted with water, producing acidic solutions
- He concluded that an acid must contain oxygen. For example:
- $\ce{SO2 +H2O->H2SO3}$
- $\ce{CO2 +H2O ->H2CO3}$
Davy - 1810 - Hydrogen
- English chemist Humphry Davy (famous for his redox and electrolytes works) demonstrated that hydrochloric acid did not contain oxygen, thus disproving Lavoisier’s theory.
- Davy suggested that hydrogen must be the unifying component of acids rather than oxygen.
Liebig - 1838 - Acid + Metal
- The German chemist Justus von Liebig expanded on Davy’s idea. In 1838, he proposed that acids contain hydrogen which could be displaced by a reaction with a metal. For example:
- $\ce{Mg +HCl ->MgCl2 +H2}$
- $\ce{Zn +H2SO4 ->ZnSO4 +H2}$
Arrhenius - 1884 - $\ce{H+}\text{ and }\ce{OH-}$
- Svante Arrhenius proposed the first concept of acids and bases we still use
- His work centered on the conductivity of electrolytes
- His theory was that electrolytes dissociated in water in ions
- He defined acids and bases according to their effect in water
- An Arrhenius Acid is a substance that produces a Hydrogen Ion when dissolved in water, for example:
$$\ce{HCl_{(aq)}->H+{(aq)} +Cl-{(aq)}}$$
- Arrhenius also notated that the most reactive acids also had the highest electrical conductivities
- This has led to the concept that the strongest acids are the most dissociated in an aqueous solution
- An Arrhenius Base is a substance which produces $OH^{-}$ when dissolved in water.
Limitations of the Arrhenius Models
- Arrhenius’ definition does not explain the basic behaviour of substances like ammonia, which do not contain hydroxide ions in their formulae and hence should not be able to produce $OH^-$
- It does not explain why neutralisation reactions between some acids and bases produced solutions that were not neutral
- The reaction between ammonia and hydrochloric acid produces an acidic solution. ($\ce{NH4Cl}$ – an acidic salt)
- The reaction between acetic acid and sodium hydroxide produces a basic solution ($\ce{NaCH3OO}$ – a basic salt)
- The Arrhenius definition only covers acids and bases in aqueous solutions
Bronsted-Lowry Acids and Bases
- In 1923, Danish chemist Johannes Nicolaus Bronsted and English chemist Thomas Martin Lowry independently proposed a new definition of acids and bases.
Acids and bases are defined by their role in a reaction:
The proton donor is the Bronsted-Lowry acid
The proton acceptor is the Bronsted-Lowry base
The Bronsted-Lowry definition allows many more species to be defined as acids or bases. It can explain the basic behaviour of ammonia. The $\ce{NH3{(aq)}}$ is accepting a proton from $\ce{HCl}$ in the aqueous solution. $\ce{NH3{(aq)}}$ is a Bronsted Lowry base and $\ce{HCl}$ is a Bronsted Lowry acid.
- Bronsted Lowry theory also explains the basic behaviour of ionic compounds in solution.
- Soluble carbonates and hydrogen carbonates contain Bronsted Lowry bases. They produce basic solutions.
First the compound dissolves in water to produce aqueous ions. This step proceeds completely, since all Group 1 ionic compounds are soluble:
$\ce{Na2CO3 → 2Na+ + CO3^2−}$
The dissolved carbonate or hydrogen carbonate ion is a Bronsted Lowry base which reacts with water to produce hydroxide ions:
$\ce{CO3^2- +H2O<=>HCO3- +OH-}$
The Bronsted Lowry definition is broad enough that some species like water can be classified both an acid and a base.
For example, when ammonia dissolves in water, water donates H+ to ammonia and is acting as a Bronsted Lowry acid: $\ce{NH3 +H2O <=>NH4+ +OH-}$
When $HCl$ dissolved in water, water accepts the hydrogen ion, so Hydrochloric acid becomes a Bronsted-Lowry Base: $\ce{HCl +H2O->H3O+ +Cl-}$
Substances which can both donate and accept protons are known as Amphiprotic Substances
Hydronium Ions
- A $\ce{H+}$ ion is a bare proton with a $1+$ charge. This means that any $\ce{H+}$ ion in water immediately combines with a water molecule to form a more stable hydronium ion, $\ce{H3O+}$
- $\ce{H+}$ does not technically exist independently in solution.
$$\color{orange}{\ce{H+ +H2O->H3O+}}$$
Strengths of Acids and Bases
Strong/Weak Acids
- A strong acid is a substance which ionises completely in water (static equilibrium) to produce hydronium ions in aqueous solutions
- Strong acids include:
- $\ce{HCl}$
- $\ce{HNO3}$
- $\ce{H2SO4}$
- $\ce{HBr}$ (Hydrobromic Acid)
- $\ce{HClO4}$ (Perchloric Acid)
- $\ce{HClO3}$ (Chloric Acid)
- Most other common acids are weak acids, which ionide partially in water (dynamic equilibrium) to form hydronium ions in solution
Acid Dissociation Constant $(K_{a})$
$$K_a = \frac{[\ce{H+}][\ce{A-}]}{HA}$$
- The higher the $K_a$, the stronger the acid strength, as it favours the right hand side
- Most acids are monoprotic: they can only give 1 proton. However, some acids can give more than 1 proton, and are known as polyprotic acids
- Polyprotic acids have multiple $K_a$ values, depending on how many protons they give up
- For example, Phosphoric acid is a triprotic acid:
| Reaction | $K_a$ |
|---|---|
| $\ce{H3PO4<=>H+ +H2PO4-}$ | $K_{a1}=\frac{[H^+][\ce{H2PO4-}]}{[\ce{H3PO4}]}=7.52\times 10^{-3}$ |
| $\ce{H2PO4-<=>H+ +HPO4^2-}$ | $K_{a2}=\frac{[H^+][\ce{HPO4^2-}]}{[\ce{H2PO4-}]}=6.23\times 10^{-8}$ |
| $\ce{HPO4^2-<=>H+ +PO3^3-}$ | $K_{a3}=\frac{[H^+][\ce{PO4^3-}]}{[\ce{HPO4^2-}]}=2.20\times 10^{-13}$ |
- The number of protons an acid produces is unrelated to its strength
Strength and concentration are not the same, nor are they dependent on each other:
- Strength depends on the identity of the acid/base and the extent of its ionization in water
- Concentration depends on the amount of the substance in a given solution
Conjugate Acids and Bases
The ionisation of an acid can be represented by the following equation:
$$\ce{HA(aq)<=>H+(aq) +A-(aq)}$$
- When the equation is read in reverse, it shows the $A-$ accepting a proton (and thus acting as a Bronsted-Lowry Base)
- $HA$ and $A^-$ are referred to as a conjugate acid/base pair
- The difference between the two species is a proton $(\ce{H+})$
- When the acid loses a proton, it forms the conjugate base
- When the base gains a proton, it forms the conjugate acid
- An acid’s conjugate base has 1 less proton, while a base’s conjugate acid has 1 more proton
- Acid→Donates $\ce{H+}$→Conjugate Base
- Base→Accepts $\ce{H+}$→Conjugate Acid
Relative Strengths of Conjugate Pairs
- The two species in a conjugate pair have inverse strength: $\require{ams}$
- A strong acid will have an extremely weak (virtually neutral) conjugate base (e.g. $\ce{HCl}$ and $\ce{Cl-}$), and vice versa
- A strong acid has an equilibrium which lies far to the right: in essence, it has virtually no reverse reaction:
$$\begin{gather*}\bbox[5px, border: 2px solid orange]{\bbox[5px, border: 2px solid red]{\text{Strong BL Acid}}\text{ + Water}}\rightarrow\bbox[5px, border: 2px solid pink]{\text{Hydronium +}\bbox[5px, border: 2px solid green]{\text{Weak BL Base}}} \\ \bbox[5px, border: 2px solid orange]{\bbox[5px, border: 2px solid red]{\ce{HCl(aq)}} \ce{+H2O(l)}}\ce{->}\bbox[5px, border: 2px solid pink]{\ce{H3O+(aq) +}\bbox[5px, border: 2px solid green]{\ce{Cl-(aq)}}} \\ \bbox[5px, border: 2px solid orange]{0\text{%}}\longrightarrow \bbox[5px, border: 2px solid pink]{100\text{%}}\end{gather*}$$
- A weak acid has an extremely strong conjugate base, and so the equilibrium lies far to the left
$$\begin{gather*}\bbox[5px, border: 2px solid orange]{\bbox[5px, border: 2px solid red]{\text{Weak BL Acid}}\text{ + Water}}\rightarrow\bbox[5px, border: 2px solid pink]{\text{Hydronium +}\bbox[5px, border: 2px solid green]{\text{Strong BL Base}}} \\ \bbox[5px, border: 2px solid orange]{\bbox[5px, border: 2px solid red]{\ce{HF(aq)}} \ce{+H2O(l)}}\ce{->}\bbox[5px, border: 2px solid pink]{\ce{H3O+(aq) +}\bbox[5px, border: 2px solid green]{\ce{F-(aq)}}} \\ \bbox[5px, border: 2px solid orange]{92\text{%}}\longrightarrow \bbox[5px, border: 2px solid pink]{8\text{%}}\end{gather*}$$
Practice Question 1
Explain why the presence of Nitrate ions in an aqueous solution will not make it basic (i.e. will not produce extra $\ce{OH-}$ ions). (3 Marks)
$$\ce{HNO3(aq) +H2O(l) ->H3O+(aq) +NO3-(aq)}$$
Note: The reaction can be considered to go to completion, because the reverse reaction does not occur to any significant extent.
Toggle Solution
- Identify that $NO^-_3$ is a neutral ion
- Identify that it does not form Hydroxide ions
- Identify that without hydroxide ion formation, the solution cannot be basic
EXAMPLE ANSWER: $NO^-_3(aq)$ has virtually no tendency to accept hydrogen ions, and is thus a neutral ion (1). As a result, it does not accept any $\ce{H+}$ from water to produce $\ce{OH-}$ ions (2). Since no hydroxide ions are formed, the solution does not become basic by the addition of Nitrate ions (3).
Applying Brönsted-Lowry Theory
Non-Neutral Salt Solutions
Many ions are not neutral when dissolved in water. As a result, they will have either acidic or basic properties.
- This means that in neutralisation reactions, the salt product is not necessarily neutral
- The pH depends on the nature of the salt
To show that an ionic compound will form an acidic, basic, or neutral solution:
- Write an equation showing the dissociation of the compound into its two ions
- Determine if either ion is acidic or basic by looking at their conjugates:
- Strong acid + Weak base → Acidic
- Strong acid + Strong base → Neutral
- Weak acid + Strong base → Basic
- Write an equation showing the acidic or basic ion reacting with water to form $\ce{H3O+}$ or $\ce{OH-}$
Example Question
Will a solution of potassium fluoride be acidic or basic? (2 Marks)
$$\ce{KF(aq)->K+(aq) +F-(aq)}$$
Toggle Solution
- Identify Fluoride as a strong conjugate base
- Write the equation showing the formation of $\ce{OH-}$
EXAMPLE ANSWER:
$\ce{F-}$ is the conjugate base of a weak acid $(\ce{HF}),$ and will therefore be a strong base. As a result, it will have a high tendency to react with water to form $OH^-$, resulting in a basic solution of $pH\gt7.$
$$\ce{F-(aq) +H2O(l)<=>OH-(aq) +HF(aq)}$$
Amphiprotic Substances
Water is both a Brönsted-Lowry Acid and base, and is therefore an amphiprotic substance
Other amphiprotic substances include $\ce{HCO3-,HSO4-,H2PO4-,HPO4- \text{ and }NH3}$
Amphiprotic substances are a subset of amphoteric substances, which can react with both acids and bases
In laboratories, Sodium Bicarbonate $(\ce{NaHCO3(s)})$ is commonly used for neutralising acid spills as it is a weak, amphoteric base that produces $\ce{CO2}$ during neutralisation
- As a weak base, it is not very exothermic
- As an amphoteric substance, it will absorb extra $\ce{OH-}$ if too much is added
- As a producer of $\ce{CO2},$ it has a visible product when neutralisation is complete
- As a solid, it does not contribute to the size of the spill
Acid/Base Behaviour of Oxides
An acidic oxide is one which either reacts with water to form an acidic solution or reacts with bases to form acidic salts. Common acidic oxides are $\ce{CO2}$ and $\ce{P4O10}$ (diphosphorus pentoxide) and $\ce{SO2}$.
$$\ce{CO2(g) +H2(l)<=>H2CO3(aq)<=>H+(aq) +HCO3-(aq)}$$
$$\ce{CO2(g) +2NaOH(aq) ->Na2CO3(aq) +H2O(l)}$$
Non-metal from the RHS of the periodic table tend to form acidic oxides. These elements have high electronegativity and share electrons when bonding with oxygen, so non-metal oxides are covalent.
A basic oxide is one that reacts with water to form an alkaline solution or reacts with acids to form basic salts. Metals from the LHS of the periodic table tend to form basic oxides. These elements have low electronegativity, so metal oxides are ionic.
pH Scale
PH AND $H^+$ CONNECTION
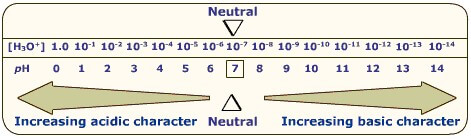
- pH 7 is neutral at 25℃ (Concentration of hydroxide and hydronium ions are equal)
The pH (potential of hydrogen) scale is logarithmic (base 10), not linear. Significant figures for logs are those after the decimal point.
$$pH=-\log_{10}[\ce{H+}]$$
PH OF STRONG AND WEAK ACIDS
pH can deduce the relative strength and concentration of different acidic solutions.
↑ Acid strength → ↑ Degree of ionisation → ↑ [$H^+$] → ↓ pH
↑ [Acid] → ↑ [$H^+$] → ↓ pH
Acid strength and concentration both affect the pH of a solution.
For a monoprotic strong acid, the concentration of $\ce{H+}$ is the same as the concentration of the acid $(𝐻𝑋 → 𝐻^+ + 𝑋^−)$ For weak acids, the concentration of $H^+$ will depend on its strength and concentration of the acid solution.
$𝐻𝐴 ⇌ 𝐻^+ + 𝐴^−$Where $ K_a = \frac{[\ce{H+}][\ce{A-}]}{HA}$
POH OF STRONG AND WEAK BASES
pOH is a measure of $OH^-$ concentration that is similar to pH.
- $p$$𝑂𝐻 = − log_{10}[𝑂𝐻^−]$
Strong bases are Group 1 and 2 hydroxides. The concentration of hydroxide ions depends on the number of hydroxide ions in the formula.
For weak bases, the concentration of $OH^-$ will depend on its strength and the concentration of the base solution.
AUTOIONISATION OF WATER
The concentration of $\ce{H+}$ and $\ce{OH-}$ in any aqueous solution are directly related. This is because water is a very weak acid which forms the following exothermic equilibrium reaction.
- The equilibrium constant for this equilibrium is called the ionisation constant for water.
$$\begin{gather*}\color{orange}{K_w=\ce{[H3O+][OH-]}} \\ \color{orange}{=1.0\times10^{-14}} \\ \color{orange}{pK_{w}=pH+pOH =14} \\ \color{orange}{pH=14+\log_{10}[\ce{OH-}]}\end{gather*}$$
$pK_{a}$ AND $pK_{b}$
Since$K_{a}$ values are usually very small, $pK_{a}$ values are often cited instead:
$p$$K_{a}$ = − $\log_{10}$ $K_{a}$
$K_{a}$ = 10−$p$$K_{a}$
↑ Strong acid → ↑$K_{a}$ → ↓$pK_{a}$
PH MEASUREMENTS
PH PROBE
A pH probe or pH meter can be used to measure pH.
Advantages: Precision, non-destructive
Disadvantages: Initial costs, requires calibration before use
PH OF MIXED SOLUTIONS
DILUTION CALCULATIONS
The total moles of solute in a concentrated solution and the diluted solution are the same.
- $c_{1}\cdot v_{1}=c_{2}\cdot v_{2}$
Practice Question
Calculate pH when 1.0 mL of 1.0M HCl is diluted to 1.25L.
Toggle Solution
$c_{1}\cdot v_{1}=c_{2}\cdot v_{2}$
$c_{2}$ = = 0.0008 𝑚𝑜𝑙/𝐿
[𝐻𝐶𝑙] = $\ce{[H+]}$= 0.0008 𝑀
$pH$ = −log$\ce{[H+]}$= − log(0.0008) = 3.10 (2 sig. figures)
BUFFERS
HOW DO BUFFERS WORK?
A buffer solution is a solution that can resist pH change when small amounts of an acid or base are added. In order for it to work, it must be able to compensate for the addition of either of an acid or base, otherwise the pH will change significantly.
Buffers are commonly made by mixing a weak acid and its conjugate base (or vice versa) typically in equimolar amounts of each.
- For example, a buffer solution containing $\ce{CH3COOH}$ and $\ce{CH3COO-}$ (in the form of $NaCH_3COO$) - The mixture exists in equilibrium, so all of the species in the equation are present.
$\ce{CH3COOH(aq) +H2O(l)<=>CH3COO-(aq) +H3O+(aq)}$
When a small amount of acid is added, the concentration of $\ce{H3O+}$ increases.
When HCl is added to the buffer solution, ↑ $\ce{H3O+}$ which is a disturbance
It will shift to minimise the disturbance by shifting to the LHS and forming the products.
Role of conjugate base is to react with excess $\ce{H3O+}$. It ‘absorbs’ the additional $\ce{H3O+}$
When a small amount of base is added, the situation is slightly more complicated.
There are two acids that could react with the base $\ce{CH3COOH}$ and $\ce{H3O+}$
Because the concentration of $\ce{H3O+}$ is far lower than the concentration of $\ce{CH3COOH}$, the majority of $\ce{OH-}$ will react with $\ce{CH3COOH}$, producing water and $\ce{CH3COO-}$
Thus the concentration of $\ce{H3O+}$does not change significantly, and the pH stays relatively constant.
It can also be explained when $\ce{H3O+}$ and $\ce{OH-}$ react, causing equilibrium to shift to right to minimise the disturbance
$\ce{H3O+(aq) +OH-(aq)<=>2H2O(l)}$
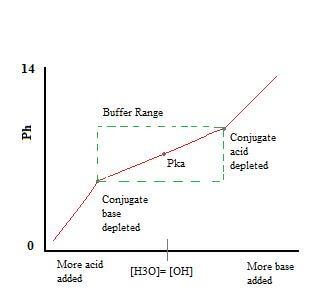
BUFFER CAPACITY
The effectiveness of a buffer is known as buffer capacity.
It is defined as the moles of $\ce{H3O+}$ or $\ce{OH-}$ necessary to change the pH of the buffer solution by one unit
Buffer capacity depends on both the pH of the buffer and the total concentration of the weak acid and conjugate base (or vice versa)
A buffer is most effective when the amounts of weak acid and conjugate base present are similar (equimolar).
When pH = $pK_{a}$, the concentrations of weak acid and conjugate base are equal.
Therefore, a buffer solution is most effective when the pH is within 1 unit of its $pK_{a}$.
When the pH is too high, there is not enough acid to react with the added $\ce{OH-}$. When the pH is too low, there is not enough conjugate base to react with any $\ce{H3O+}$.
TITRATION CALCULATIONS
DIRECT TITRATION CALCULATIONS
The steps for a titration calculation are:
Write a balanced chemical equation for the reaction
Calculate the number of moles of Reactant A (of known concentration) in the volume used
Using the number of moles of A and the mole ratio in the equation, calculate the number of moles of Reactant B (unknown concentration) used.
Calculate the concentration of reactant B.
For neutralisation reactions, the strength of the acid is irrelevant as the base is stronger than water. All of the protons in a polyprotic acid will be irreversibly removed by the base.
DILUTION - TITRATION CALCULATIONS
When analysing a substance, the concentration may be too high for a direct titration experiment tom be efficiently carried out. Instead, the substance would be diluted by a known amount, and then the diluted solution would be titrated.
BACK TITRATION CALCULATIONS
A back titration, or indirect titration, is a two-stage analysis:
Reactant A (of unknown concentration) is reacted with an excess of Reactant B (of known concentration and volume).
A titration is the performed on the excess Reactant B by determining the moles of Reactant C required to neutralise the excess.
Summary:
Sample is reacted with known excess of reagent. (e.g. known amount of a particular acid)
Leftover excess is added
Excess is titrated to find moles of reagent reacted with solution.
Back titrations are generally used when:
One of the reactants is volatile (e.g. ammonia)
An acid or base is an insoluble salt (e.g. calcium carbonate)
Direct titration would involve weak acid/weak base titration (making it difficult to determine the equivalence point).
TITRATION
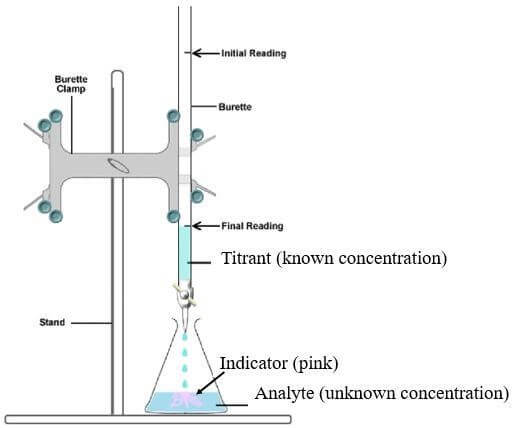
TITRATION TERMINOLOGY
Titration Terminology
Titration - Volumetric analytical technique used for determining the concentration of a solution, when the concentration of the other reacting solution is known and volumes of the 2 solutions are accurately measured.
Aliquot - An accurately known volume of liquid. In titration this usually refers to the liquid transferred via pipette into the conical flask.
Analyte A - substance being analysed. This is usually a solution of unknown concentration.
Back Titration - A two-stage analysis in which an excess of reactant is added to the analyte, then the excess is determined to calculate the concentration of the analyte.
Burette - A graduated piece of glassware which dispenses measured amounts of the solution (the titrant).
Endpoint - The point in titration when the indicator permanently changes colour.
Equimolar - Same concentration.
Equivalence Point - The point in a neutralisation reaction when the amounts of reactants are just sufficient to consume both reactants, without an excess of either. (i.e. in stoichiometric ratio)
Pipette - A piece of glassware used to transfer a very accurately measured volume of solution.
Primary standard - A substance of sufficiently high purity and stability that a solution of accurately known concentration can be prepared by weighing out the desired mass, dissolving in water and making the volume up to a known value.
Titrand - The solution to which another reagent (titrant) is added during titration (usually in the conical flask)
Titrant - The solution that is added during a titration (usually from a burette)
Titre - The volume of titrant used in a titration
Volumetric flask - A piece of glassware which can hold a set volume of solution very accurately
TYPES OF ANALYSIS
- Qualitative analysis: Involves observations only
- Quantitative analysis: Involves measurements (mass, volume etc.)
- Volumetric analysis: involves measurements of volume
- Gravimetric analysis: involves measurements of mass/weight
GENERAL TITRATION PROCEDURE
In a titration experiment, the number of moles of a target material (the analyte) is determined. This can then be used to calculate the concentration.
A measured volume of the solution of unknown concentration, the analyte, is usually placed in a conical flask (titrand) with a burette containing the titrant above it.
A burette is a piece of volumetric glassware. It is a long tube with a tap at one end so measured volumes of titrant can be accurately added to the titrand.
Before the experiment begins, an indicator will normally also be added to the conical flask to determine the approximate equivalence point.
To perform the experiment the titrant is slowly added and stepwise to the conical flask, with swirling, until the indicator undergoes a permanent colour change (the end point).
Acid-base titrations are the most common titrations:
An acid and a base are reacted in a neutralisation reaction during the titration.
A suitable acid-base indicator is added to show when the reaction is just complete. However, indicators change colour over a range of pH, making it difficult to accurately determine the equivalence point. - For greater accuracy, a pH meter can be used.
The pH changes rapidly towards the end point of the titration and it is easy to add too much titrant. The titrant must be added very carefully, in small volumes close to the end to successfully determine the exact amount required for complete reaction.
DETAILED TITRATION PROCEDURE
For titrating HCl against NaOH. HCl is placed into the conical flask and NaOH in the burette.
PREPARING THE TITRAND (IN THE CONICAL FLASK)
Rinse the inside of the pipette with a small with a small amount of HCl solution 3 times.
Use a pipette filler to fill the pipette with HCl until the bottom of the meniscus rests on the calibration line.
Hold the pipette so that its tip is resting against the inside of a clean conical flask. Let the solution run out.
Once the liquid level has stabilised, leave the pipette tip touching the flask for a few seconds before removing. (The pipette is calibrated to deliver the correct volume when a small amount of liquid remains in the tip – do not shake it into the flask).
Use a wash bottle containing distilled water to wash any solution that might be on the inside wall to the bottom of the conical flask.
PREPARING THE TITRANT (IN THE BURETTE)
Rinse the inside of the burette with a small amount of NaOH solution 3 times, including through the tap.
Clamp the burette vertically
Pour NaOH solution into the burette
TITRATION
Add a few drops of indicator into the conical flask and swirl gently.
Record the initial burette reading
Place the conical flask under the burette
Add NaOH to the conical flask until a permanent colour change occurs
Record the final burette reading
Repeat the experiment 3 times with fresh aliquots of HCl.
WASHING
Burette and pipette: Used to deliver the solutions used in the titration. Final rinsing with the solution to be delivered.
Conical flask: Used to hold the aliquot or titrand. Final rinsing with distilled water.
The washing procedure affects the accuracy of the calculated concentration.
TITRATION TECHNIQUE
RINSING
It is important to rinse each piece of glassware with the appropriate solution after cleaning with distilled water and immediately prior to use.
The solution that is to be transferred using a pipette is of accurately known concentration, or its concentration is to be accurately determined. If droplets of distilled water are present in the pipette, it will dilute the reagent being delivered.
Rinse burette and pipette with solutions
Rinse conical flask and volumetric flask with distilled water
VOLUMETRIC ERRORS
All glassware in titration is calibrated to be accurate when measurements are taken at the bottom of the meniscus.
Any air bubbles in the liquid must be removed for volumes to be accurate.
The pipettes used in titration are calibrated to deliver the specified volume of solution with no additional force.
They are marked TD (to deliver) or EX (to expel)
This means that there should be a small volume of liquid left in the tip of the pipette after the aliquot has been accurately transferred. This should not be shaken out into the conical flask.
Assessing accuracy - How close you are to the accepted value:
$$\color{orange}{\text{% deviation}=\frac{\text{|experimental value - accepted value|}}{\text{accepted value}}}$$
STANDARD SOLUTION
A standard solution is a solution containing a precisely known concentration of a substance. They can be categorised as primary or secondary.
A primary standard is produced when a substance of high purity dissolved in a known volume of solvent.
Accurately weigh out a mass of solid close to the required mass in a beaker. Record the actual mass weighed.
Add enough distilled water to dissolve the solid.
Carefully transfer all the weighed mass to a clean volumetric flask of the approximate size, using a wash bottle and funnel. All the equipment that came into contact with weighed mass should be rinsed into the flask.
Add distilled water until the bottom of the meniscus is resting on the line on the neck of the flask. Add the last few drops with a dropper.
Stopper the flask. Firmly holding the stopper in place, invert several times to ensure the solution is homogeneous.
Label the flask with the exact concentration, solution, date and name.
A substance suitable for preparing a primary standard solution should have the following features:
- High purity
- Unaffected by exposure to air
- Non-hygroscopic (does not absorb water from air)
- Have a large molecular mass to reduce percentage errors
- Be a solid for easier weighing
- Cheap and readily available
- Have a high water solubility
A secondary standard is produced when its concentration is determined via stoichiometry.
- The process of producing a secondary standard is called standardisation.
EQUIVALENCE POINT
The equivalence point of a titration is the point at which the amount moles of acid and bases added match the stoichiometric ratio.
It is the point at which reaction is complete, with no excess reactant
The pH of the solution at the equivalence point determines the appropriate indicator to be used.
PH OF THE EQUIVALENCE POINT
As the pH of water is neutral, the pH of the equivalence point will depend entirely on the salt produced: whether it is acidic, basic or neutral.
If an acidic or basic salt is produced by the neutralisation reaction in a titration experiment, the equivalence point will not be neutral.
Neutral salts are formed when strong acids react with strong bases.
Acidic salts are formed when strong acids react with weak bases. - Basic salts are formed when weak acids react with strong bases.
Note: When strong acids react with metal carbonates (weak bases), the neutral salt is formed, but the resulting solution is still acidic. This is because the carbon dioxide dissolves in water to produce an acidic solution.
INDICATOR SELECTION
Indicators can be used to find the approximate equivalence point of a titration.
The equivalence point is when exactly enough moles of titrant have been added to react with all the titrand.
The end point is when the indicator first undergoes a permanent colour change.
An indicator should be selected so that the end point is as close as possible to the equivalence point. (Systematic error. Will impact validity and accuracy)
Indicator Color at Low pH Color at high pH Color Change pH Range Methyl Orange Red Yellow 3.1-4.4 Bromothymol Blue Yellow Blue 6.0-7.6 Phenolphthalein Colorless Pink 8.3-10.0 Methyl Red Red Yellow 4.8-6.0 Litmus Red Blue 4.5-8.3 Phenol Red Yellow Red 6.8-8.4
BROMOTHYMOL BLUE
Explain why 0.20 M acetic acid and 0.20 M hydrochloric acid require the same volume of sodium hydroxide solution to reach equivalence point, but the pH values at their equivalence points are different.
𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞) + 𝑁𝑎𝑂𝐻(𝑎𝑞) → 𝐶𝐻3𝐶𝑂𝑂𝑁𝑎(𝑎𝑞) + 𝐻2𝑂(𝑙)
𝐻𝐶𝑙(𝑎𝑞) + 𝑁𝑎𝑂𝐻(𝑎𝑞) → 𝑁𝑎𝐶𝑙(𝑎𝑞) + 𝐻2𝑂(𝑙)
𝐶𝐻3𝐶𝑂𝑂(−𝑎𝑞) + 𝐻2𝑂(𝑙) ⇌ 𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞) + 𝑂𝐻(−𝑎𝑞)
Both acetic acid and hydrochloric acid are monoprotic acids that reacts to completion when reacted with a strong base. As the concentration are the same, the same amount is needed to neutralise the strong base, NaOH. $\ce{CH3COO-}$ is the conjugate base of a weak acid and will react with water to produce a basic solution. Therefore, resulting in a pH > 7. $\ce{Cl-}$ is the conjugate base of a strong acid and will not react hence the solution remains pH = 7.
APPLICATION OF TITRATION
The equivalence point is located at the most vertical point (point of inflection). All three common indicators for titration are suitable for determining the equivalence point for a strong acid-strong base. This is because there is a large rapid change in pH near the equivalence point so all of the indicator would change colour when the same volume of based is added, therefore it is not critical which indicator is used.
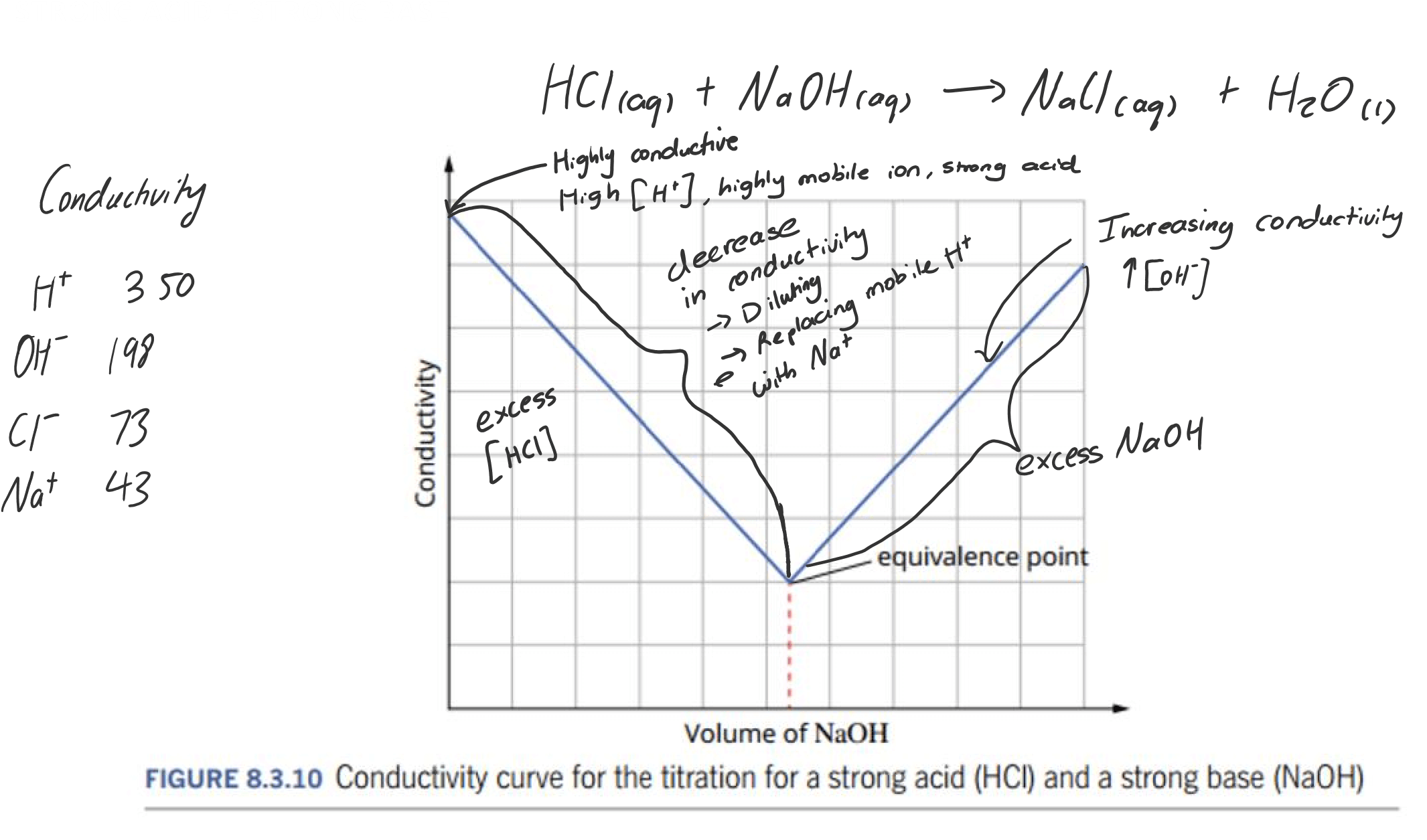
CONDUCTIVITY GRAPHS
During a titration, the conductivity of the solution changes. The equivalence point may be located by plotting the conductance as a function of the volume of titrant added.
The electrical conductivity of a solution depends on:
- The concentration ions present
- The mobility of the ions present:
- More mobile ions, the more conductive it is $\ce{H+}$ and $\ce{OH-}$ are highly mobile
- $\ce{H+}$ ions are more conductive than $\ce{OH-}$ ions
Conductometric titrations are useful for titrations of coloured solutions, analysis of dilute solutions, and when reversible reactions are used (e.g. weak acid-weak base titration).
General rule:
Strong → Linear
Weak → Curved
WEAK ACID + STRONG BASE

Conductivity is initially low as acetic acid is a weak acid and only partially ionises in water.
[Not shown on the graph] As the base is initially added, conductivity decreases
- $\ce{H+}$ is replaced by $\ce{Na+}$ which is less conductive
- The presence of the newly formed acetate ions also decreases the ionisation of acetic acid due to the common ion effect.
𝐶𝐻3𝐶𝑂𝑂𝐻(𝑎𝑞) + 𝑁𝑎𝑂𝐻(𝑎𝑞) → 𝐶𝐻3𝐶𝑂𝑂𝑁𝑎(𝑎𝑞) ⇌ 𝐶𝐻3𝐶𝑂𝑂(−𝑎𝑞) + 𝑁𝑎(+𝑎𝑞)
The initial production of the $\ce{CH3COO-}$ ions results in the suppression of the $\ce{CH3COOH}$ ionisation due to the common ion effect.
- Conductivity then increases are more $\ce{Na+}$ and $\ce{CH3COO-}$ are produced.
- There is a minimal change in pH due to the buffer region. The mixture of $\ce{CH3COO-}$/$\ce{CH3COOH}$
Reaches the equivalence point
Conductivity increases more rapidly as $\ce{Na+}$ and highly conducting $\ce{OH-}$ are added. (Excess strong base)
Initially, the conductance is low due to the low ionisation of the weak acid. On the addition of the strong base, there is a decrease in conductance due to the replacement of the $\ce{H+}$ by $\ce{Na+}$ but also supresses the dissociation of the acetic acid due to the common ion acetate.
The conductance increases on adding $\ce{NaOH}$ as it neutralises the undissociated $\ce{CH3COOH}$ to $\ce{NaCH3COO}$ which is a strong electrolyte. Conductivity increases due to the highly conductive $\ce{OH-}$ ions.
STRONG ACID + WEAK BASE

$\ce{NH4+(aq) +H2O(l)<=> NH3(aq) +H3O+(aq)}$
Before the equivalence point, conductivity decreases like in the strong acid-strong base graph.
After the equivalence point, the graph is almost horizontal as the excess weak base is not significantly ionised due to the presence of its conjugate acid.
Initially, the conductance is high due to the strong acid. The conductance decreases due to the replacement of $\ce{H+}$. After the equivalence point has been reached in the graph becomes almost horizontal, since the excess weak base
(aqueous ammonia) is not easily ionised in the presence of the salt.
WEAK ACID + WEAK BASE
$\ce{CH3COOH}$ is a weak acid and therefore only partially ionises.
[$\ce{H+}$] gets used up. $\ce{CH3COO-}$ gets produced which suppresses the ionisation of $\ce{CH3COOH}$.
Production of more ions
Equivalence point
Excess $\ce{NH3}$ is suppressed due to the common ion effect