Physical Properties
- Elements are pure substances made up of one type of atom that can’t be broken down into simpler substances.
- Compounds are pure substances made up of 2 or more different types of atoms and can be decomposed by heat, electrolysis, and high energy EM radiation.
- Compounds can exist in 3 ways:
- Ionic compounds (salts) - A metallic element bonded to one or more non-metallic elements forming a crystal lattice through a complete transfer of electrons.
- They don’t exist as discrete molecules but in a ratio of atoms.
- Covalent compounds - A group of 2 or more non-metal atoms bonded by sharing electrons to form discrete molecules.
- Lattice/Network - A 3D network of atoms chemically bonded in a lattice that are not discrete molecules.
- Ionic compounds (salts) - A metallic element bonded to one or more non-metallic elements forming a crystal lattice through a complete transfer of electrons.
- Mixtures are composed of non-chemically bonded substances and can either be:
- Homogeneous - Components are uniformly mixed and have one phase e.g. solutions, alloys, unsaturated salt water.
- Alloys: Mixture of two or more elements with at least one of them being a metal.
- Alloys have different properties to the metals which they contain.
- This makes them more useful than pure metals.
- They are often harder than the metals they contain e.g. steel, brass, bronze, stainless steel.
- Alloys: Mixture of two or more elements with at least one of them being a metal.
- Heterogeneous - Not uniformly mixed and can have more than one phase e.g. muddy water, sand, rocks.
- True solutions: made where a solute is dissolved in a solvent.
- Suspensions: where the solute does not dissolve in the solvent. The solute particles will settle and can be filtered out e.g. $\ce{CaCO3}\newcommand{deg}{^{\circ}}$ in water.
- Colloids: particles are dispersed throughout but are not heavy enough to settle.
- The particles are not as small as a solution but are smaller than those of a suspension.
- They can be separated by filtering e.g. milk, mayonnaise, paint, fog.
- Homogeneous - Components are uniformly mixed and have one phase e.g. solutions, alloys, unsaturated salt water.
- Physical changes only affect the form of the substance
- Substance changes its physical appearance but not composition
- Changes are reversible
- Involves the breaking of intermolecular forces but not bonds
- Particles stay the same but move differently
- A small amount of heat or energy may be taken in or given out
- Chemical changes affect the chemical composition of the substance
- A new substance is formed
- A gas may be given off
- There may be a change in colour
- There may be a change in temperature
- A precipitate may form
- Changes are irreversible
- Standard Temperature and Pressure (STP): $0\deg C,\newcommand{orange}{\color{orange}}$ 100kPa
- Standard Laboratory Conditions (SLC): $25\deg C,\newcommand{pink}{\color{pink}}$ 100kPa
Unless given otherwise.
Separation Techniques and Their Properties
| Separation Method | Property which enables separation |
|---|---|
| Sieving | Particle size |
| Evaporation | Liquid has a lower boiling point than solids |
| Distillation | Relatively large differences in boiling point between two substances |
| Fractional Distillation | Small differences in boiling point between two substances usually in the same phase |
| Separating funnel | Components are immiscible and have different densities |
| Filtration | One solid, the other a liquid or solution |
| Sedimentation | Density |
Quantitative vs Qualitative Analysis
- Qualitative analysis determines what substances are present
- Quantitative analysis determines how much of each substance is present
Percentage Composition
$\orange{\text{Percentage Composition}=\frac{\text{Mass of component}}{\text{Total mass of Mixture}}\times 100}$
Schrodinger’s Theory of the Atom
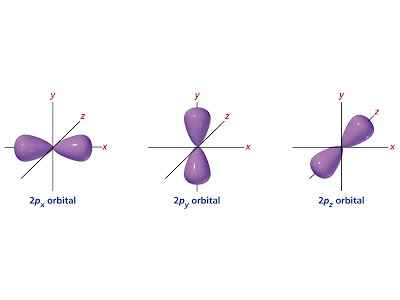
- Orbitals are geometric areas of 95% electron presence probability as determined by Schrodinger’s equation in which one or two electrons can move.
- Principal energy levels (1, 2, 3…) are groupings of similar energy sublevels (s, p, d, f) which are in turn groupings of individual orbital lobes.
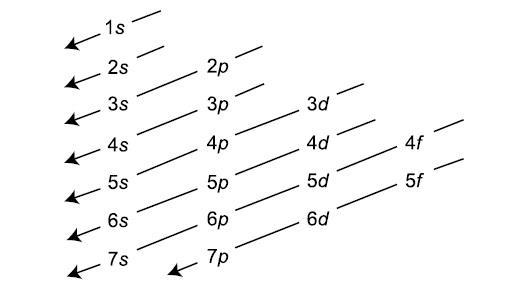
- The Aufbau principle governs that electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels
- Exceptions to Aufbau principle occur in the d block due to the reduced energy distinction between s and d levels and Coulombic repulsion (incl. Cu, Ag, Cr)
- The Heisenburg uncertainty principle states that the position and momentum of a particle (such as an electron) can not be measured accurately simultaneously
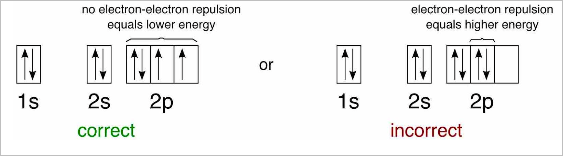
- The Pauli exclusion principle and Hund’s rule states that filled first by electrons with up spin, and then are completed with electrons of down spin (identical particles cannot occupy the same quantum state within a system)
Valency
Valence electrons are the electrons present in the outermost shells of an atom
Elements in the same group have the same number of valence electrons
Valency is the maximum number of direct bonds formable with the element.
Variable valency may occur when an atom loses or gains more electrons than the valence shell.
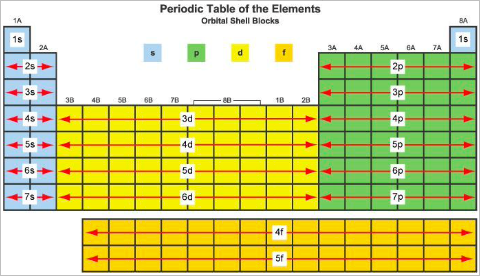
Orbital notation can be determined by filling to the appropriate orbital given the number of electrons or through the use of spdf blocks on the periodic table by counting the number of filled orbitals.
- Helium and groups 1 and 2 comprise s orbitals from energy levels 1-7
- Transition metals comprise d orbitals from energy levels 3-6
- Excluding helium, groups 13 - 18 comprise p orbitals from energy levels 2-7
- Lanthanides and actinides comprise group f from energy levels 4-5
Atomic Spectra and Flame Tests
When electrons are supplied with energy they can become excited.
De-excitation causes the release of energy.
The re-emission of energy occurs as different wavelengths of light.
Flame test: visible spectrum of light indicates ion
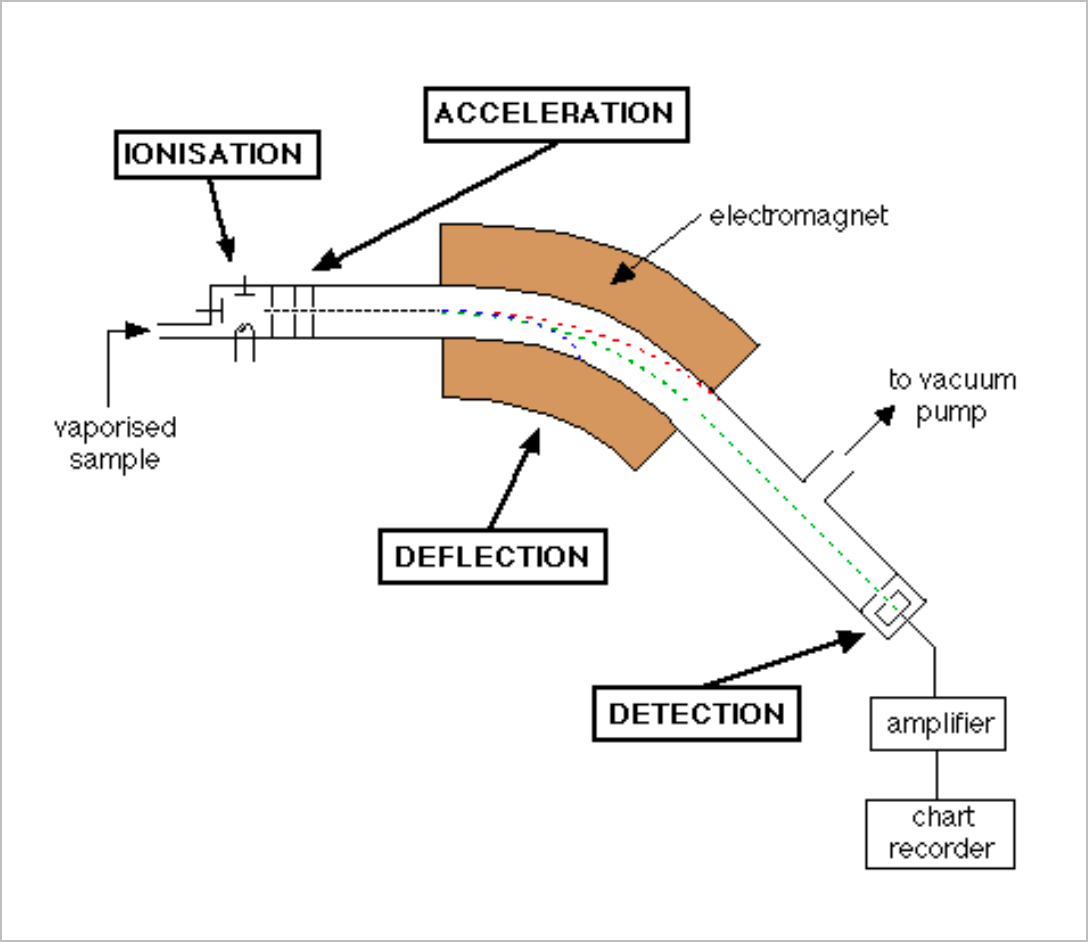
Mass spectrometry analyses the spectrum of light released by means of dispersion through an electromagnetic lens which is detected by an amplifier (which only identifies matching elements)
Isotopes and Radioactive Chemistry
Isotopes are elements with the same number of protons, but different numbers of neutrons $\newcommand{orangebox}{\bbox[5px, border: 2px solid orange]}\newcommand{pinkbox}{\bbox[5px, border: 2px solid pink]}\newcommand{greenbox}{\bbox[5px, border: 2px solid green]}$
Isotopes are named by their $\orangebox{\text{element}},$ and distinguished by their $\pinkbox{\text{Hadron}}$ (protons + neutrons) count:
- A carbon atom with 8 neutrons is called $\orangebox{\text{Carbon}}-\pinkbox{14}$
- This can be generalised to $\orangebox{\text{Element Name}}-\pinkbox{\text{Hadron Count}} $
When represented symbolically, Isotopes take the form $^{\bbox[5px, border: 2px solid pink]{A}}_{\bbox[5px, border: 2px solid green]{Z}}\bbox[5px, border: 2px solid orange]{M}$ where A is the mass number, Z is the atomic number, and M is the element’s symbol
Ionizing Radiation
- There are 4 main types of radiation:
- Alpha $(\alpha)$ radiation is an emission of the particle $^\pinkbox{{4}}_\greenbox{{2}}\orangebox{He}$ $\left(\orangebox{Helium}-\pinkbox{4}\right)$ which has a charge of 2+, and a mass of 4 AMU
- Alpha radiation can be blocked by paper, and most other materials
- Beta $(\beta)$ radiation is the emission of an electron $^\pinkbox{{0}}\orangebox{e}^{-1}$ or positron $^\pinkbox{{0}}\orangebox{e}^{1+}$
- Because they are electron particles, they have negligible mass $\left(\frac{1}{2000} AMU\right)$
- However, they also have a charge, which means they are likely to be repelled by metallic or charged materials (such as aluminum or lead)
- Gamma $(\gamma)$ radiation consists of high energy electromagnetic radiation with no charge or mass
- Gamma radiation is only blocked by thick layers of concrete or lead
- Neutron radiation is a high energy neutron
- Neutron radiation has high mass, but no charge, meaning it can penetrate multiple layers of lead/concrete (because of the high particle momentum)
- However, it tends to be absorbed by boron and cadmium compounds (which are dissolved in water; this is why nuclear reactors are surrounded by water)
- Alpha $(\alpha)$ radiation is an emission of the particle $^\pinkbox{{4}}_\greenbox{{2}}\orangebox{He}$ $\left(\orangebox{Helium}-\pinkbox{4}\right)$ which has a charge of 2+, and a mass of 4 AMU
Relative Atomic Mass
To calculate relative atomic mass:
$$\orange{RMS=\sum{(\text{Relative Abundance of Isotope}\times\text{Mass of Isotope})}}$$
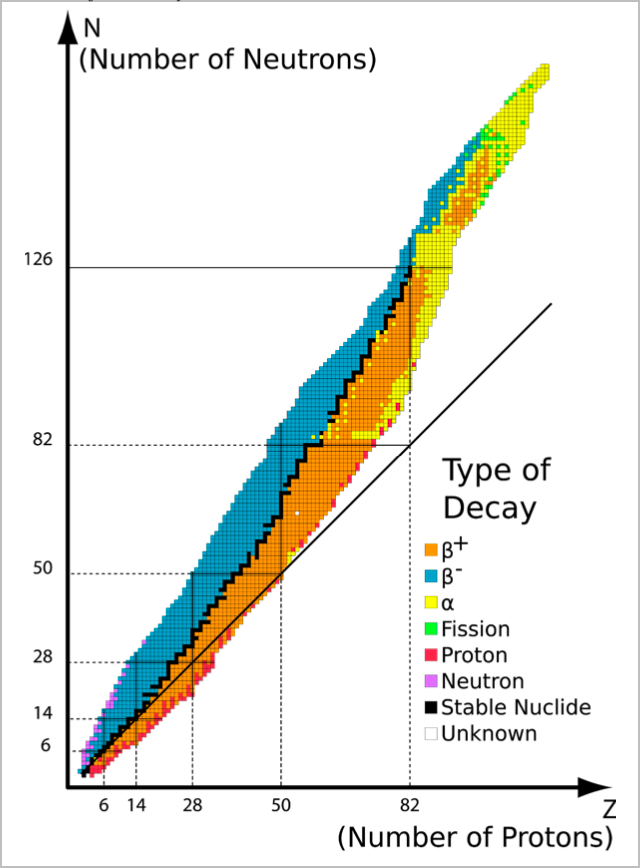
Isotope Stability
Periodicity:
Increasing from the given corner:
Upper-right → lower left:
- Atomic radius
- Metallic character
Lower-left → upper right:
- Ionisation energy - The energy needed to remove an electron from a gaseous atom
- Electron affinity
- Electronegativity - The ability of an atom to attract electrons
- Non-metallic character
Core charge (the number of valence electrons) increases across a period
Reactivity increases from the upper right corner of s, d, f-blocks and from the lower left corner in p block, excepting noble gases
Shielding increases with period
Melting point and boiling point increase until across the period from group 1 to group 14, then drastically drops for groups 15-18. M.p. and B.p. decrease down a group, suggesting that metallic bonds in crystals of metals are weaker as atoms get larger.
Valency +1 for group 1, +2 for group 2, variable for d-block, and +3, -4, -3, -2, -1, 0 for p-block
- 1, 2, 3, transitions, 4, 3, 2, 1, 0
Chemical Bonding and Intermolecular Forces
Allotropes
- Carbon has several allotropes, which are forms of the element as a molecule, with different physical properties:
- Diamonds have a high refractive index, are extremely hard, and have a covalent lattice structure
- Graphite has carbon atoms bonded in flat hexagonal rings in layers, which are extremely strong in one direction, but slide easily in the other
- Fullerenes $(\ce{C60})$ are a soccer-ball pattern structure of 60 carbon atoms
Polarity and Intermolecular Forces
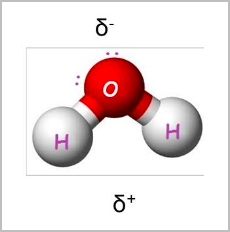
- Polarity means there is a net difference in charge between two areas in a molecule: If two bonded atoms are different, the electrons will stay closer to the more electronegative atom (has stronger pull).
- This produces a small charge imbalance, making one end of the bond more negatively charged δ− than the other δ+ .
- The difference in electronegativity can lead to three outcomes:
- No difference $\rightarrow$ Nonpolar Covalent
- Moderate difference $\rightarrow$ polar covalent
- Very different $\rightarrow$ ionic bond
Types of intermolecular forces list by strength in descending order:
Ionic bonding commonly occurs between negatively and positively charged ions (sodium chloride crystals)
Ion-dipole interactions occur between polar molecules and charged ions (sodium ions in water)
Hydrogen bonding occurs when electropositivity induced (from F, N, O) on a hydrogen ion leads
to dipole-dipole-type interactions.For hydrogen bonding to occur, a hydrogen atom must be bonded to three very electronegative atoms which are F, N and O. This creates a large partial positive charge on the hydrogen and is attracted to the lone pairs on nitrogen, oxygen or fluorine. Hydrogen bonds are 10 times stronger than dipole-dipole bonds but only 1/10th the strength of an ionic or covalent bond. Hydrogen bonding is present in compounds such as water and ethanoic acid. The density of ice is less than liquid water as when water freezes, it forms a lattice, with molecules being held further apart than in liquid.
Dipole-dipole interactions occur in molecules that have a net dipole such as in polar molecules (NH3 - NH3, ICl - ICl). These forces result from the electrostatic attraction between the positive and negative ends of two or more dipoles. These forces are relatively weak as the partial charges are small. However, the more polar a molecule is, the stronger the dipole-dipole forces will be.
● Ion-induced dipole interactions occur when a relatively stronger ion induces the polarisation of an electron cloud in another molecule (Fe2+ ion - dioxygen)
Dipole-induced dipole interactions occur where a polar molecule with dipole moment asymmetricality induces the polarisation of an electron cloud in another molecule (HCl - dichloride)
Dispersion forces occur when momentary dipoles are formed. Weakest of all IMFs. This temporary charge can influence a change in the charge distribution in nearby molecules. This creates a temporary charge between the negative end and a positive end of two different molecules. There are billions of these interactions being made and broken every second. The strength of a dispersion force increases as the size of the molecule increases as more electrons will increase the probability of temporary dipoles.
Types of Molecular Structures
Crystalline Structures are usually solid, non-volatile, and have high melting points
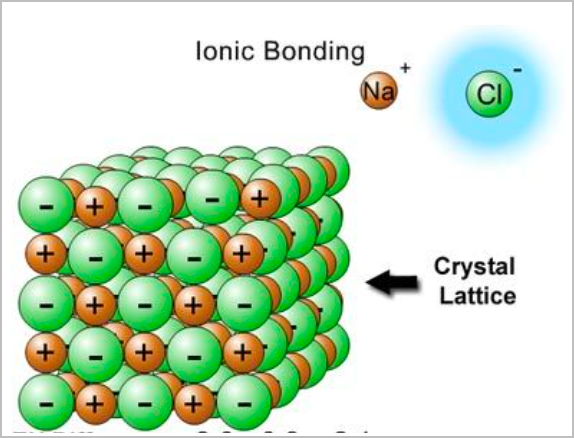
- An attraction and combination between a cation and an anion will form an ionic lattice network.
- They are large structures that naturally exist due to intermolecular electrostatic attractions.
- They conduct electricity when molten or aqueous as for substances to conduct electricity, they must have charged particles that are free to move.
- Ionic compounds are hard. There are no discrete molecules and their formulas are empirical.
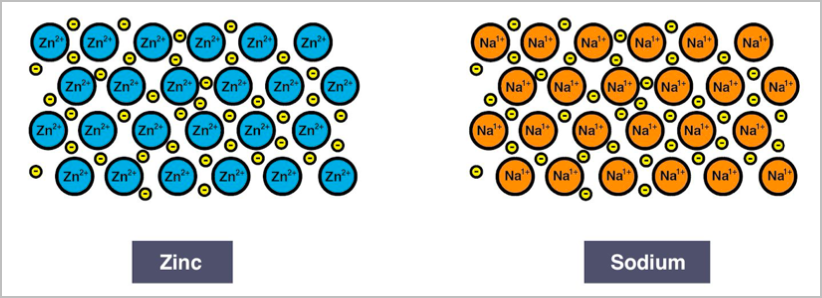
- Metallic networks have a sea of delocalised electrons surrounding a positively charged cation with electrostatic bonding and good conductivity of electricity and heat.
- The electrostatic attraction between the cations and delocalised electrons is called metallic bonding.
- They have lustre and are malleable. E.g. zinc and sodium
- Covalent lattice networks have covalent bonding throughout the structure and are similar to ionic lattice but have higher melting points and generally do not conduct electricity e.g. Diamond, Graphite, Silicon Dioxide, Silicon Carbide. They are of tetrahedral shape.
- Covalent molecular compounds are usually amorphous liquids or gases, volatile, have low boiling points, are poor conductors, and are soft.
Molecular geometry:
The octet rule states that atoms generally combine to obtain the noble gas configuration (8 valence electrons). A share of 8 valence electrons is the most stable arrangement of electrons, however it doesn’t apply to certain molecules including BF3, PCl5 and SF6. VSEPR is a theory used to predict the geometries of molecules with a central atom around which bonding occurs by modelling the repulsion of valence shell electrons from each other
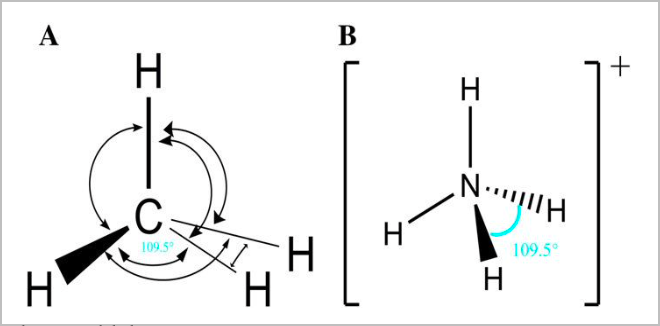
● Tetrahedral:
- This is the most stable way for four equal electron clouds to arrange themselves
- There are 4 bonding pairs, and no lone pairs [4,0]
- 109.5 degree angle between atoms
- E.g. NH4, CH4, SiCl4, CCl4
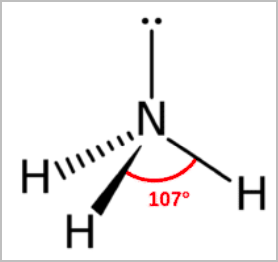
- Trigonal pyramidal:
- Tetrahedral arrangement of electron pairs without one of the atoms.
- Has 3 bonding pairs and 1 lone pair [3,1]
- 107 degree angle between atoms
- E.g. NH3, PCl3
- Bent shape:
- Tetrahedral arrangement of electron pairs but only two are bonded.
- 2 bonding pairs and 2 lone pairs [2,2]
- 105 degree angle between atoms
- E.g. H2O, H2S, CH2
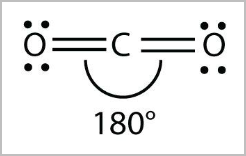
- Linear:
- Tetrahedral arrangement of electron pairs but only one bonding pair, or 2 bonding pairs with no lone pairs ( CO2, HCN, BeH2 ) [2,0] [2,3]
- 180 degree angle
- E.g. HCl, HF
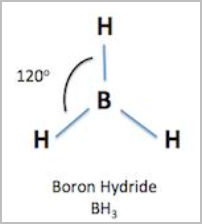
- Trigonal planar:
- Repulsion between electron pairs is minimised by a triangular arrangement about the central atom such that the electron pairs are in the same place.
- 3 bonding pairs and no lone pairs [3,0]
- 120 degree angles between atoms
- E.g.BH, BF, SO, NO3
Lewis diagrams
- Dots on lewis diagrams represent the number of valence electrons in the given atom (octet rule generally states a full shell is 8 valence electrons)
- For ionic compounds, bracket the ion structure with the charge
- Line bonds represent covalent bonds only
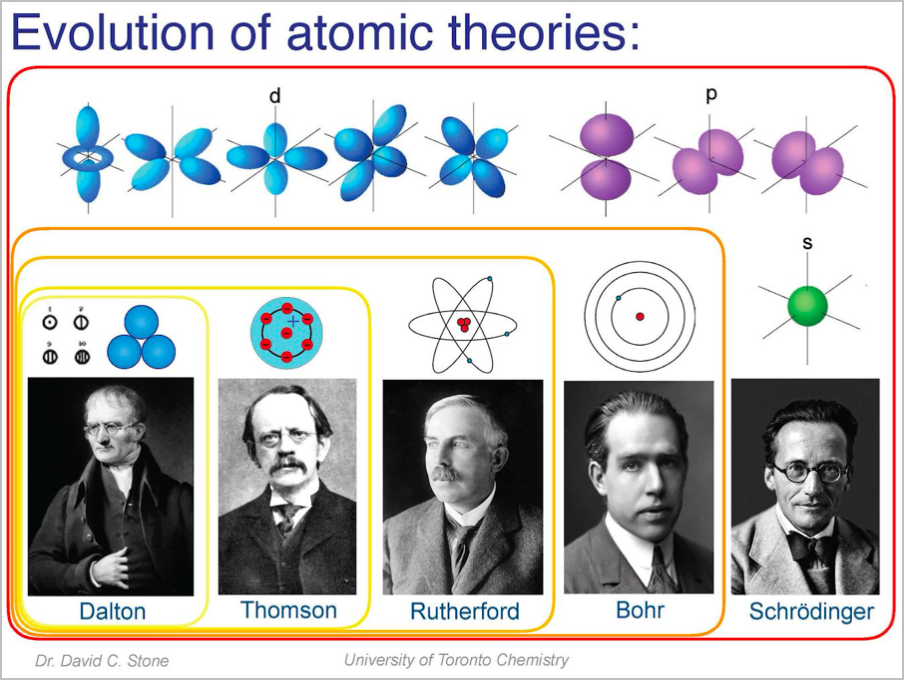
Evolution of Atomic Theories
- Democritus: There is a smallest size which things can be reduced to
- Dalton: Different elements have atoms of differing size and mass
- Thomson: Plum pudding model - small electrons in a large area of positive charge
- Rutherford: Atom has a positively charged nucleus (gold foil experiment); electrons orbit the nucleus
- Chadwick: Discovery of neutrons in the nucleus
- Bohr: Electrons exist in discrete energy levels
- Schrodinger: Quantum/geometric model of electron position (orbitals)